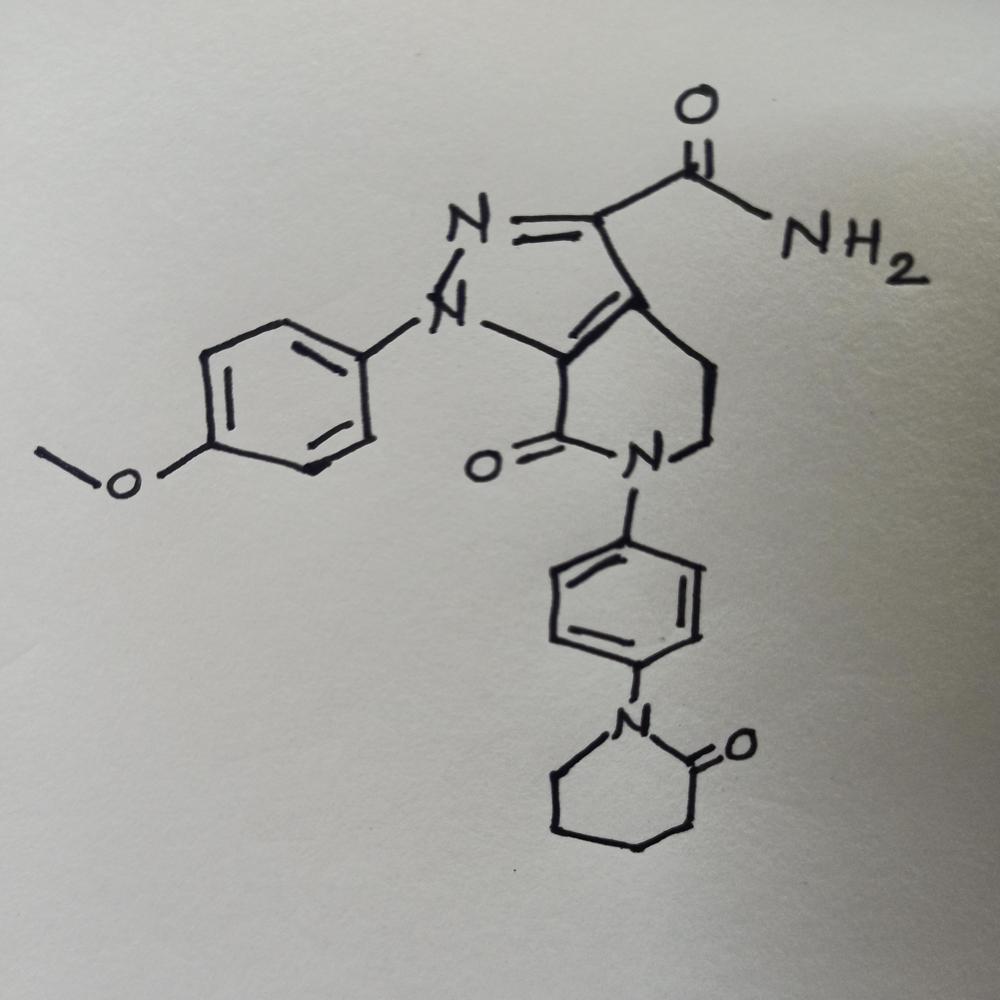
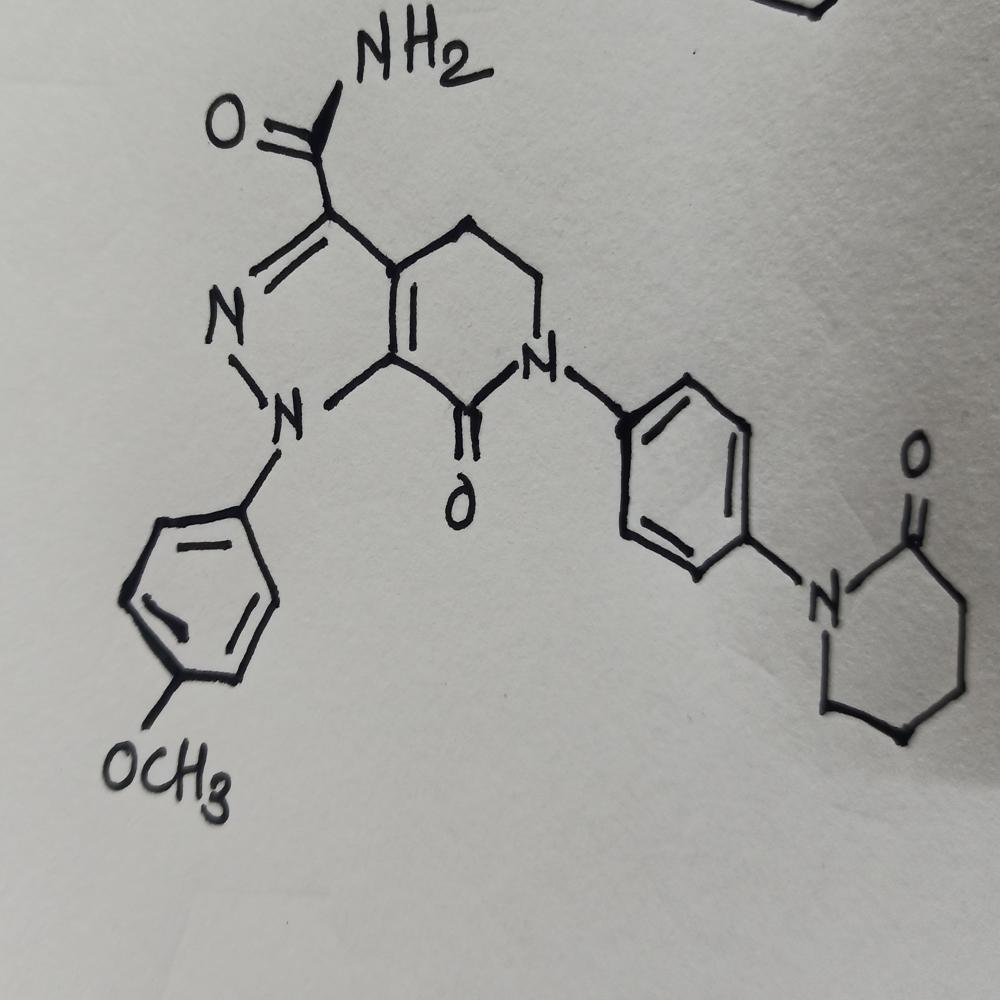
Apixa-ban Powder Api CASNo. 503612-47-3 Specification
- Molecular Weight
- 459.5 Grams (g)
- Molecular Formula
- C25H25N5O4
- Type
- Pharmaceutical Intermediates
- Appearance
- white to pale yellow powder
Apixa-ban Powder Api CASNo. 503612-47-3 Trade Information
- Minimum Order Quantity
- 50 Kilograms
- Payment Terms
- Cash Advance (CA)
- Main Export Market(s)
- Asia
- Main Domestic Market
- All India
- Certifications
- AVD Pharmaceuticals Pvt. Ltd. is ISO certified. 9001:2015 certified for Quality Management system. Manufacturer of speciality chemicals, Drugs Itermediate, Pharmaceutical raw material.
About Apixa-ban Powder Api CASNo. 503612-47-3
Apixaban is recommended by the National Institute for Health and Clinical Excellence for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation and at least one of the following risk factors: prior stroke or transient ischemic attack, age 75 years or older, diabetes, or symptomatic heart failure.[17]Apixaban and other anticoagulants (dabigatran, edoxaban and rivaroxaban) appear equally effective as warfarin in preventing non-hemorrhagic stroke in people with atrial fibrillation and are associated with lower risk of intracranial bleeding.[1819]While apixaban may be used in people with severely decreased kidney function and those on hemodialysis it has not been studied in these groups.